Retention in reversed-phase LC can be predicted by the linear solvent strength model (5) in which the logarithm of the retention factor of the solute is inversely proportional to the percentage of the strong solvent. Fischer, G. Goldberg, and P.C. The same separation and detection conditions were applied as described in Figure 6. Selection of Organic Solvent or Mobile Phase B in Reversed-Phase LC. The internal filters in most HPLC pumps (replaced during preventive maintenance programs) appear to be adequate to allow successful routine HPLC operation without filtering in most laboratories. Another approach that has emerged is the use of additives to mobile phase A to increase, or rescue, ionization efficiency lost to trifluoroacetic acid ionization suppression. The use of ion-pairing reagents has decreased somewhat in recent years, because of issues such as lower column efficiencies, slow column equilibration, difficulties in performing gradient analysis, and the lack of MS-compatibility (1,2). Note that availability of high-pH-compatible volatile mobile phases (such as ammonium hydroxide), with suitable silica-based or hybrid columns, can offer a compelling alternative MS-compatible approach for the analysis of water-soluble basic drugs, and thereby reduce the utility of need for ion-pairing or chaotropic reagents for such separations (15). Toxicol.19, 233242 (1994). The Use of Mobile Phase B Containing Water and Additives. Newer additives (difluoroacetic acid and 3-fluoropropionic acid) as alternatives to trifluoroacetic acid for an increased MS sensitivity are suggested. Before the 1990s, silica-based columns could not be used with high pH mobile phases, because of the dissolution of silica support at pH values >8. Recent research by Boyes and associates has uncovered two promising additives that deliver a good compromise of performance for peak shape and MS sensitivity: difluoroacetic acid and 3-fluoropropionic acid (26). Note the excellent peak shapes of all peaks under high-pH conditions without any peak splitting.
Also, there can be secondary interactions, such as those between basic functional groups undergoing ionic strength-dependent interactions with free residual silanols present on stationary phase surfaces, which can result in tailed peaks (7). Comparisons of peptide separations with inline MS detection shows trifluoroacetic acid strongly suppressed ionization, difluoroacetic acid much less so, with formic acid or ammonium formate exhibiting highest signal, whereas trifluoroacetic acid, difluoroacetic acid, and ammonium formate show excellent peak shape. Figure 4: Chromatograms illustrating gradient shift problems encountered during method development of a stability-indicating method for a drug substance using (a) 0.1% trifluoroacetic acid in mobile-phases A and B and (b) balanced absorbance mobile phases of mobile-phase A of 0.05% trifluoroacetic acid in water and mobile-phase B of 0.03% trifluoroacetic acid in acetonitrile. Column: 150 mm x 3.0 mm, 3.5-m Waters XTerra MS C18; mobile-phase A: 20 mM ammonium bicarbonate, pH 9.1; mobile-phase B: acetonitrile; gradient: 350% B in 40 min, 5080% B in 3 min; flow rate: 1 mL/min; temperature: 50 C. He holds a PhD in Analytical Chemistry from City University of New York. However, the widespread availability of improved ion chromatography instruments with suppressed conductivity or MS detection has eliminated most of these approaches with limited applicability. Higher concentrations can be used effectively to increase retention in reversed-phase LC by the "salting out" effect or the Le Chatelier's Principle to drive solutes into the hydrophobic stationary phase (1,11). 2022 MJH Life Sciences and Chromatography Online. Rev.16(4), 36-43 (2013). (28) H. Hahne, F. Pachl, B. Ruprecht, S.K. 30), such as the case of charged surface hybrids (CSH) introduced by Waters in 2010 (13). This elimination of the filtration step can reduce potential mobile phase contaminations from the filtration process. With suitable care, the correct composition of mobile phase A can be maintained, with minimal risk of introducing contaminants that can readily appear in high sensitivity analyses. Proceedings ASMS, St. Louis MO (2015), ID 234745. Squire and J.M. Snyder and J.W. Methanol has substantial UV end-absorbance below 210 nm. A,779(1), 29-71 (1997). Paradoxically, innovation in reversed-phase LC centers on new instruments and columns while scant research efforts are placed on the development of newer reagents for mobile phases. Retention is proportional to the length of the hydrophobic chain of the ion-pairing agent and its concentration of the ion-pairing reagent. (14) D. Johnson, B.E. (20) M.W. Adapted with permission from reference 20. The elimination of the filtration process and many less relevant mobile phase additives is also favored. Separation of proteins and peptides is a challenging application for HPLC requiring bonded phases with low silanophilic activity, specific mobile-phase additives to allow detection at low UV wavelengths (210220 nm), and higher column temperatures (>60 C) to improve peak shapes, and mass recovery (2, 3). in Biochemistry at the University of Alberta, then M.Sc. Commonly used acids are trifluoroacetic acid, formic acid, and acetic acid at concentrations of 0.05 to 0.1% v/v. Robust stability-indicating methods for very complex pharmaceuticals can be accomplished by using multisegment binary linear gradients (23). Gradient: 2565% B in 15 min; baseline shift: (a) 0.1 AU, (b) 0.002 AU; detection: UV absorbance at 230 nm. Boyes, W. Miles and B. Libert, J. Biomol. A,1217(6), 858880 (2010). The addition of such compounds does not hinder effective LC performance and has been shown to restore signal intensities heavily suppressed by trifluoroacetic acid. For instance, a mobile phase of 44% methanol:water was found to have equivalent elution strength of 35% acetonitrile:water or 28% tetrahydrofuran:water for a reference application (11). The C18 column yielded excellent symmetrical peaks for all NCEs when used with a buffered mobile-phase A (such as 20 mM ammonium formate at pH 3.7).

Adapted with permission from reference 3. Buffers of ammonium salts of volatile acids are used for the development of MS-compatible HPLC methods. It is UV-transparent to 200 nm, but it is not volatile, and thereby not MS-compatible. Over more than six years of use, difluoroacetic acid has shown no negative effects on instrument performance in standard or capillary column separations. Changing mobile phase B to 85% acetonitrile in water or methanol can alleviate this issue. Although quaternary pumps are widely used for method development, quality control methods that use ternary or quaternary mobile phases are rarely encountered, because of the difficulties with method reproducibility across different laboratories or equipment platforms. Fountain, H.B. Glajch, J.J. Kirkland, K.M.
and Ph.D. degrees in Neuroscience at UBC (Vancouver, Canada). Kazakevich and R. LoBrutto, Eds., HPLC for Pharmaceutical Scientists (Wiley, Hoboken, New Jersey, 2007), Chapter 4. Mass Spectrom. A common MS-compatible buffer system is 20 mM ammonium formate adjusted to pH 3.7 with formic acid. The ghost peak was later identified to be a preservative (sodium phenylphenate) used in the pH calibration buffers. 31(6), 472-479 (2013). Because of the absorbance of trifluoroacetic acid in the far UV region (200230 nm), significant baseline shift (~0.1 AU at 230 nm) was observed. An acidic pH of 24 is used for most pharmaceutical applications. Column: 50 mm x 3.0 mm, 1.7-m Waters BEH C18. Obviously, fresh mobile phases can be prepared daily as needed, although this time-consuming process is neither a necessary or "green" practice. Figure 6 illustrates the comparative performance of four mobile phase additives (formic acid, ammonium formate/formic acid, trifluoroacetic acid, and difluoroacetic acid) in the HPLCUVMS analysis of a mixture of five peptides. A buffer is prepared by mixing a weak acid with the salt of its conjugate base (or a weak base with the salt of its conjugate acid). The disadvantage of this method is its sensitivity to mobile phase pH which must be kept between 9.10 to 9.15 to prevent any peak co-elution (3,15). (29) M. Nshanian, R. Lakshmanan, H. Chen, R. R. Ogorzalek Loo, and J.
(5) L.R.
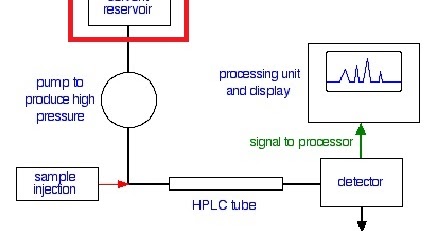
The potentials for growth of microorganisms (bacteria, algae, molds) poses the greatest risks to system contamination and column longevity. It is readily prepared and is transparent down to 200 nm. J. All rights reserved. The role of the mobile phase in controlling retention and selectivity in reversed-phase LC for neutral and ionizable solutes has been discussed extensively in textbooks, and the reader is referred to them for a detailed discussion (13). (7) D. V. McCalley, J. Chromatogr. Basic analytes are ionized at low pH, though most basic drugs have sufficient hydrophobicity for adequate reversed-phase LC retention. Mobile phase B is the conventional term for the strong mobile phase (organic solvent) in reversed-phase LC used in pump blending and gradient elution. Chetwyn, LCGC North Am.


Figure 2 further illustrates the effect of mobile-phase pH to dramatically alter the retention and elution orders of acidic and basic drugs (17). Methanol is a protic solvent and functions as a proton donor or proton acceptor. Here, we discuss those and other trends and best practices, as well as the fundamentals behind them. Kind, J. Anal. Thus, the pH of mobile phase A must be controlled, as it can have a dramatic effect on solute retention. Barry has published more than 85 peer-reviewed papers, reviews, patents, and applications.
 Also, there can be secondary interactions, such as those between basic functional groups undergoing ionic strength-dependent interactions with free residual silanols present on stationary phase surfaces, which can result in tailed peaks (7). Comparisons of peptide separations with inline MS detection shows trifluoroacetic acid strongly suppressed ionization, difluoroacetic acid much less so, with formic acid or ammonium formate exhibiting highest signal, whereas trifluoroacetic acid, difluoroacetic acid, and ammonium formate show excellent peak shape. Figure 4: Chromatograms illustrating gradient shift problems encountered during method development of a stability-indicating method for a drug substance using (a) 0.1% trifluoroacetic acid in mobile-phases A and B and (b) balanced absorbance mobile phases of mobile-phase A of 0.05% trifluoroacetic acid in water and mobile-phase B of 0.03% trifluoroacetic acid in acetonitrile. Column: 150 mm x 3.0 mm, 3.5-m Waters XTerra MS C18; mobile-phase A: 20 mM ammonium bicarbonate, pH 9.1; mobile-phase B: acetonitrile; gradient: 350% B in 40 min, 5080% B in 3 min; flow rate: 1 mL/min; temperature: 50 C. He holds a PhD in Analytical Chemistry from City University of New York. However, the widespread availability of improved ion chromatography instruments with suppressed conductivity or MS detection has eliminated most of these approaches with limited applicability. Higher concentrations can be used effectively to increase retention in reversed-phase LC by the "salting out" effect or the Le Chatelier's Principle to drive solutes into the hydrophobic stationary phase (1,11). 2022 MJH Life Sciences and Chromatography Online. Rev.16(4), 36-43 (2013). (28) H. Hahne, F. Pachl, B. Ruprecht, S.K. 30), such as the case of charged surface hybrids (CSH) introduced by Waters in 2010 (13). This elimination of the filtration step can reduce potential mobile phase contaminations from the filtration process. With suitable care, the correct composition of mobile phase A can be maintained, with minimal risk of introducing contaminants that can readily appear in high sensitivity analyses. Proceedings ASMS, St. Louis MO (2015), ID 234745. Squire and J.M. Snyder and J.W. Methanol has substantial UV end-absorbance below 210 nm. A,779(1), 29-71 (1997). Paradoxically, innovation in reversed-phase LC centers on new instruments and columns while scant research efforts are placed on the development of newer reagents for mobile phases. Retention is proportional to the length of the hydrophobic chain of the ion-pairing agent and its concentration of the ion-pairing reagent. (14) D. Johnson, B.E. (20) M.W. Adapted with permission from reference 20. The elimination of the filtration process and many less relevant mobile phase additives is also favored. Separation of proteins and peptides is a challenging application for HPLC requiring bonded phases with low silanophilic activity, specific mobile-phase additives to allow detection at low UV wavelengths (210220 nm), and higher column temperatures (>60 C) to improve peak shapes, and mass recovery (2, 3). in Biochemistry at the University of Alberta, then M.Sc. Commonly used acids are trifluoroacetic acid, formic acid, and acetic acid at concentrations of 0.05 to 0.1% v/v. Robust stability-indicating methods for very complex pharmaceuticals can be accomplished by using multisegment binary linear gradients (23). Gradient: 2565% B in 15 min; baseline shift: (a) 0.1 AU, (b) 0.002 AU; detection: UV absorbance at 230 nm. Boyes, W. Miles and B. Libert, J. Biomol. A,1217(6), 858880 (2010). The addition of such compounds does not hinder effective LC performance and has been shown to restore signal intensities heavily suppressed by trifluoroacetic acid. For instance, a mobile phase of 44% methanol:water was found to have equivalent elution strength of 35% acetonitrile:water or 28% tetrahydrofuran:water for a reference application (11). The C18 column yielded excellent symmetrical peaks for all NCEs when used with a buffered mobile-phase A (such as 20 mM ammonium formate at pH 3.7).
Also, there can be secondary interactions, such as those between basic functional groups undergoing ionic strength-dependent interactions with free residual silanols present on stationary phase surfaces, which can result in tailed peaks (7). Comparisons of peptide separations with inline MS detection shows trifluoroacetic acid strongly suppressed ionization, difluoroacetic acid much less so, with formic acid or ammonium formate exhibiting highest signal, whereas trifluoroacetic acid, difluoroacetic acid, and ammonium formate show excellent peak shape. Figure 4: Chromatograms illustrating gradient shift problems encountered during method development of a stability-indicating method for a drug substance using (a) 0.1% trifluoroacetic acid in mobile-phases A and B and (b) balanced absorbance mobile phases of mobile-phase A of 0.05% trifluoroacetic acid in water and mobile-phase B of 0.03% trifluoroacetic acid in acetonitrile. Column: 150 mm x 3.0 mm, 3.5-m Waters XTerra MS C18; mobile-phase A: 20 mM ammonium bicarbonate, pH 9.1; mobile-phase B: acetonitrile; gradient: 350% B in 40 min, 5080% B in 3 min; flow rate: 1 mL/min; temperature: 50 C. He holds a PhD in Analytical Chemistry from City University of New York. However, the widespread availability of improved ion chromatography instruments with suppressed conductivity or MS detection has eliminated most of these approaches with limited applicability. Higher concentrations can be used effectively to increase retention in reversed-phase LC by the "salting out" effect or the Le Chatelier's Principle to drive solutes into the hydrophobic stationary phase (1,11). 2022 MJH Life Sciences and Chromatography Online. Rev.16(4), 36-43 (2013). (28) H. Hahne, F. Pachl, B. Ruprecht, S.K. 30), such as the case of charged surface hybrids (CSH) introduced by Waters in 2010 (13). This elimination of the filtration step can reduce potential mobile phase contaminations from the filtration process. With suitable care, the correct composition of mobile phase A can be maintained, with minimal risk of introducing contaminants that can readily appear in high sensitivity analyses. Proceedings ASMS, St. Louis MO (2015), ID 234745. Squire and J.M. Snyder and J.W. Methanol has substantial UV end-absorbance below 210 nm. A,779(1), 29-71 (1997). Paradoxically, innovation in reversed-phase LC centers on new instruments and columns while scant research efforts are placed on the development of newer reagents for mobile phases. Retention is proportional to the length of the hydrophobic chain of the ion-pairing agent and its concentration of the ion-pairing reagent. (14) D. Johnson, B.E. (20) M.W. Adapted with permission from reference 20. The elimination of the filtration process and many less relevant mobile phase additives is also favored. Separation of proteins and peptides is a challenging application for HPLC requiring bonded phases with low silanophilic activity, specific mobile-phase additives to allow detection at low UV wavelengths (210220 nm), and higher column temperatures (>60 C) to improve peak shapes, and mass recovery (2, 3). in Biochemistry at the University of Alberta, then M.Sc. Commonly used acids are trifluoroacetic acid, formic acid, and acetic acid at concentrations of 0.05 to 0.1% v/v. Robust stability-indicating methods for very complex pharmaceuticals can be accomplished by using multisegment binary linear gradients (23). Gradient: 2565% B in 15 min; baseline shift: (a) 0.1 AU, (b) 0.002 AU; detection: UV absorbance at 230 nm. Boyes, W. Miles and B. Libert, J. Biomol. A,1217(6), 858880 (2010). The addition of such compounds does not hinder effective LC performance and has been shown to restore signal intensities heavily suppressed by trifluoroacetic acid. For instance, a mobile phase of 44% methanol:water was found to have equivalent elution strength of 35% acetonitrile:water or 28% tetrahydrofuran:water for a reference application (11). The C18 column yielded excellent symmetrical peaks for all NCEs when used with a buffered mobile-phase A (such as 20 mM ammonium formate at pH 3.7). 
 Adapted with permission from reference 3. Buffers of ammonium salts of volatile acids are used for the development of MS-compatible HPLC methods. It is UV-transparent to 200 nm, but it is not volatile, and thereby not MS-compatible. Over more than six years of use, difluoroacetic acid has shown no negative effects on instrument performance in standard or capillary column separations. Changing mobile phase B to 85% acetonitrile in water or methanol can alleviate this issue. Although quaternary pumps are widely used for method development, quality control methods that use ternary or quaternary mobile phases are rarely encountered, because of the difficulties with method reproducibility across different laboratories or equipment platforms. Fountain, H.B. Glajch, J.J. Kirkland, K.M.
Adapted with permission from reference 3. Buffers of ammonium salts of volatile acids are used for the development of MS-compatible HPLC methods. It is UV-transparent to 200 nm, but it is not volatile, and thereby not MS-compatible. Over more than six years of use, difluoroacetic acid has shown no negative effects on instrument performance in standard or capillary column separations. Changing mobile phase B to 85% acetonitrile in water or methanol can alleviate this issue. Although quaternary pumps are widely used for method development, quality control methods that use ternary or quaternary mobile phases are rarely encountered, because of the difficulties with method reproducibility across different laboratories or equipment platforms. Fountain, H.B. Glajch, J.J. Kirkland, K.M.  and Ph.D. degrees in Neuroscience at UBC (Vancouver, Canada). Kazakevich and R. LoBrutto, Eds., HPLC for Pharmaceutical Scientists (Wiley, Hoboken, New Jersey, 2007), Chapter 4. Mass Spectrom. A common MS-compatible buffer system is 20 mM ammonium formate adjusted to pH 3.7 with formic acid. The ghost peak was later identified to be a preservative (sodium phenylphenate) used in the pH calibration buffers. 31(6), 472-479 (2013). Because of the absorbance of trifluoroacetic acid in the far UV region (200230 nm), significant baseline shift (~0.1 AU at 230 nm) was observed. An acidic pH of 24 is used for most pharmaceutical applications. Column: 50 mm x 3.0 mm, 1.7-m Waters BEH C18. Obviously, fresh mobile phases can be prepared daily as needed, although this time-consuming process is neither a necessary or "green" practice. Figure 6 illustrates the comparative performance of four mobile phase additives (formic acid, ammonium formate/formic acid, trifluoroacetic acid, and difluoroacetic acid) in the HPLCUVMS analysis of a mixture of five peptides. A buffer is prepared by mixing a weak acid with the salt of its conjugate base (or a weak base with the salt of its conjugate acid). The disadvantage of this method is its sensitivity to mobile phase pH which must be kept between 9.10 to 9.15 to prevent any peak co-elution (3,15). (29) M. Nshanian, R. Lakshmanan, H. Chen, R. R. Ogorzalek Loo, and J.
and Ph.D. degrees in Neuroscience at UBC (Vancouver, Canada). Kazakevich and R. LoBrutto, Eds., HPLC for Pharmaceutical Scientists (Wiley, Hoboken, New Jersey, 2007), Chapter 4. Mass Spectrom. A common MS-compatible buffer system is 20 mM ammonium formate adjusted to pH 3.7 with formic acid. The ghost peak was later identified to be a preservative (sodium phenylphenate) used in the pH calibration buffers. 31(6), 472-479 (2013). Because of the absorbance of trifluoroacetic acid in the far UV region (200230 nm), significant baseline shift (~0.1 AU at 230 nm) was observed. An acidic pH of 24 is used for most pharmaceutical applications. Column: 50 mm x 3.0 mm, 1.7-m Waters BEH C18. Obviously, fresh mobile phases can be prepared daily as needed, although this time-consuming process is neither a necessary or "green" practice. Figure 6 illustrates the comparative performance of four mobile phase additives (formic acid, ammonium formate/formic acid, trifluoroacetic acid, and difluoroacetic acid) in the HPLCUVMS analysis of a mixture of five peptides. A buffer is prepared by mixing a weak acid with the salt of its conjugate base (or a weak base with the salt of its conjugate acid). The disadvantage of this method is its sensitivity to mobile phase pH which must be kept between 9.10 to 9.15 to prevent any peak co-elution (3,15). (29) M. Nshanian, R. Lakshmanan, H. Chen, R. R. Ogorzalek Loo, and J.  (5) L.R.
(5) L.R.  The potentials for growth of microorganisms (bacteria, algae, molds) poses the greatest risks to system contamination and column longevity. It is readily prepared and is transparent down to 200 nm. J. All rights reserved. The role of the mobile phase in controlling retention and selectivity in reversed-phase LC for neutral and ionizable solutes has been discussed extensively in textbooks, and the reader is referred to them for a detailed discussion (13). (7) D. V. McCalley, J. Chromatogr. Basic analytes are ionized at low pH, though most basic drugs have sufficient hydrophobicity for adequate reversed-phase LC retention. Mobile phase B is the conventional term for the strong mobile phase (organic solvent) in reversed-phase LC used in pump blending and gradient elution. Chetwyn, LCGC North Am.
The potentials for growth of microorganisms (bacteria, algae, molds) poses the greatest risks to system contamination and column longevity. It is readily prepared and is transparent down to 200 nm. J. All rights reserved. The role of the mobile phase in controlling retention and selectivity in reversed-phase LC for neutral and ionizable solutes has been discussed extensively in textbooks, and the reader is referred to them for a detailed discussion (13). (7) D. V. McCalley, J. Chromatogr. Basic analytes are ionized at low pH, though most basic drugs have sufficient hydrophobicity for adequate reversed-phase LC retention. Mobile phase B is the conventional term for the strong mobile phase (organic solvent) in reversed-phase LC used in pump blending and gradient elution. Chetwyn, LCGC North Am. 
 Figure 2 further illustrates the effect of mobile-phase pH to dramatically alter the retention and elution orders of acidic and basic drugs (17). Methanol is a protic solvent and functions as a proton donor or proton acceptor. Here, we discuss those and other trends and best practices, as well as the fundamentals behind them. Kind, J. Anal. Thus, the pH of mobile phase A must be controlled, as it can have a dramatic effect on solute retention. Barry has published more than 85 peer-reviewed papers, reviews, patents, and applications.
Figure 2 further illustrates the effect of mobile-phase pH to dramatically alter the retention and elution orders of acidic and basic drugs (17). Methanol is a protic solvent and functions as a proton donor or proton acceptor. Here, we discuss those and other trends and best practices, as well as the fundamentals behind them. Kind, J. Anal. Thus, the pH of mobile phase A must be controlled, as it can have a dramatic effect on solute retention. Barry has published more than 85 peer-reviewed papers, reviews, patents, and applications.