NMSU and the U.S. Department of Agriculture cooperating. As part of an automated cleaning operation, an alkaline detergent is regarded as one of the most efficient and cost effective ways of removing a wide variety of soils, keeping metal clean and protected. Thats why its important to understand the chemistry behind various household cleaners and how they work. Household products that are effective sanitizers include bleach and products formulated with quats, such as pine oil cleaners. After using alkaline cleaners, acetic acid can be used as a mild deliming rinsing agent. It is a base and is especially good at removing stains and dyes from textiles. Products Bespoke Delivery Work with us Site Map, T (+44) 01403 801010 Acetic acid is the acid in clear white vinegar and is a natural all-purpose cleaning agent. The soap then washes down the drain, which helps to clear up the clog as well as dissolve any other debris. Cleaning products such as oven cleaner, lye, and drain cleaners are strong alkalis. Table 3 provides a list of possible chemical bleaching agents that may appear on product labels. Warning label on household cleaning product. Out of hours enquiries welcome. Mildly acidic cleaning products include vinegar (acetic acid) and lemon juice (citric acid). Simply sprinkle a bit of baking soda onto a damp sponge and wipe down surfaces. Types of cleaning products include. Examples of Alkalis in Household Cleaners. Detergency once active wetting agents are applied to the surface, this decreases tension in the oil surface, encouraging better penetration and displacement of soil from metals. 4 Bad Things That Can Happen. Saponication where oils and fat deposits are removed from compounds by converting them into water-soluble compounds. Most phosphates in use today, referred to as complex or condensed phosphates, have a lower alkalinity than the banned phosphates. Keep your local emergency number, local ambulance number, and the local poison control center telephone numbers on or next to your phone. For example, an alkaline cleaning solution with a pH of 10 is ten times more alkaline than a cleaner with a pH of 9.. In general, acids are useful for removing water buildup, and mineral deposits such as limestone and calcium. Therefore, it is important for consumers to recognize other names for bleach. Plus, we have 2 easy DIY recipes below on making your own alkaline cleaner. Of the natural mineral salts, baking soda is the mildest, followed by borax, and washing soda is the strongest. Contents of publications may be freely reproduced for educational purposes. Follow the directions carefully. Alkaline (aka basic) cleaners are those with a pH above 7 (with 7 being neutral). The ingredients in solvents include (but are not limited to) acetones, denatured alcohols, and mineral spirits. Dispose of cloths and brushes used to apply oxalic acid. All other rights reserved. https://www.eazychem.co.uk/product/eazy-aquaclean-hd/. It is not reactive and so can be mixed with most other cleaning agents, including chlorine bleach. Remove hard water deposits on tile and tub. Conversely, the more basic a solution, the higher its pH value. By accessing or using any page on RusticWise.com, you agree that you have read, understood, and will abide by the: Privacy & Cookie Policy | Terms | Affiliate Disclosure. As an Amazon Services LLC Associates Program affiliate, we earn from qualifying purchases. Sanitizers can be used on food contact surfaces, such as dishes, eating utensils, and cutting boards. The water needn't reach the boiling point, but should be at least as hot as you might use for tea or hot chocolate. This helps keep any potential damage to a minimum. They can remove discoloration from some metals, such as aluminum, brass, bronze, and copper. She earned her B.S. And as you may know, lye + fats/oils = soap. Lets take a closer look at the differences between acids and bases for cleaning. Avoid using vinegar on porous stone countertops and on painted or sealed wooden surfaces. Pour the hot (not boiling) water into the bowl. Spray the dirty areas about every 6 to 9 inches or so, wiping well with a rag as you move along. How does an acid cleaner differ from an alkaline cleaner? Clean food spills and everyday messes (all-purpose cleaners, soaps, and detergents). Liquid dish soap and Castile soap are considered mild or neutral soap which is gentle enough to clean a wide variety of surfaces. Moderate Alkalis Ammonia is a strong, colorless gas. Baking soda is a mild alkaline powder thats a great natural cleaning ingredient. They are useful in removing tarnish from brass and copper. It is very mild, yet more acidic than vinegar or lemon juice. They are effective in destroying a wide range of harmful bacteria, viruses, and fungi. Many waxes and polishes for furniture and floors and floor wax removers contain spirit solvents. (Avoid using boiling water as it may crack the porcelain.). Additionally, commercial advertisements may exaggerate the performance of cleaning products. Along with their detergent properties, certain alkali salts have water-softening characteristics and are used in cleaning products for that purpose. Most heavy-duty cleaners are corrosive and may. See the Caution section before using. For example, a powerful acid is battery acid, which has a pH of 0. Read More Can You Put Bleach in Your Toilet Tank? degree from BYU. Heres how soaking clothes in baking soda works, plus 8 other laundry hacks! Some laundry detergents may be used for house cleaning jobs. They help water to get a hold of the grime, break it up, and wash it away. This type of cleaner is slightly corrosive and will burn skin and corrode aluminum products. Table 1. Acid-based disinfectant cleaners are very irritating to your eyes and skin and will burn your throat. Calcium carbonate is the mildest of abrasives, with the finest abrasive found in the cream form. Polish metal faucets on sinks, showers, and tubs. Liquid chlorine bleach (Figure 1) is an alkaline solution of sodium hypochlorite dissolved in water. Ammonia also is found in glass cleaners and in cleaners used for shiny surfaces, like wax cleaners. Sodium bisulfate (also known as sodium acid sulfate) can be found in toilet bowl cleaners. Scrub with brush and flush. If your toilet tank is looking bad and smelling worse, you may wonder, can you put bleach in your toilet tank? Avoid getting them on other materials since the acids may have bleaching effects, eat through metals, or etch (scratch) surfaces and porcelain enamel. Swallowing sodium hypochlorite can lead to poisoning. The information on the label will help the physician give prompt and proper treatment. Add baking soda to a spray bottle. Use this simple, effective, and non-toxic alkali solution to clean stainless steel and/or chrome surfaces. Liquid dishwashing detergent is safe for use around children and pets. Use an acidic cleaner to do the following: In general, alkalis are useful for fighting fat and grease. University of Arkansas (Division of Agriculture), Clean and Green Homemade Cleaners. University of Florida, BASIC ELEMENTS OF EQUIPMENT CLEANING AND SANITIZING IN FOOD PROCESSING AND HANDLING OPERATIONS. Always read the labels on the products you buy and follow the directions to ensure your own safety. In commercial products, hydrochloric acid is also called muriatic acid and is used for cleaning concrete; the acid cleans the concrete by etching away the top layer. Mix until dissolved. As well as being a tried and tested method for removing moist soils, they can also help prevent metal oxidation. Please take the proper safety precautions when handling powerful cleaning substances. The safety of a laundry detergent depends on the brand and the additional chemicals it may contain. Pine Sol is an example of a pine oil cleaner/disinfectant. Remove baked on fat, oil, or grease particles. The product can be used for cleaning vegetable, animal and mineral oils/petroleum products after cargo discharge and is ideal for removing inert gas soot deposits (IGS) from cargo tanks. Chemical Bleaching Agents Found on Product Labels. Use the baking soda box as a fridge freshener. Figure 2. A strong alkaline cleaner is one with a higher pH with powerful grease and fat removing propertiesthink oven cleaners or drain cleaners. It is difficult to identify these substances because manufacturers are allowed to label these substances as confidential business information (CBI). See the Caution section before using. Revised and electronically distributed September 2012, Las Cruces, NM. Lye emits dangerous fumes and can cause skin burnsand in some cases blindnessif the fumes come in contact with your eyes.
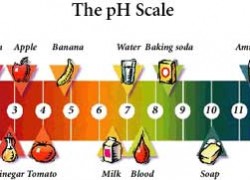
Marketing and Communications | MSC 3K, P.O. In commercial products, phosphoric acid is found in tub, tile, sink, and toilet bowl cleaners. It is environmentally sensitive, does not contain petroleum solvents and is suitable for hydrocarbon-free cleaning. When hes not tinkering in the garden, or fixing something around the house, you can find him working on a vast array of random side projects. Table 2. Rosehips are full of vitamin C and antioxidants and are used for a wide range of medicinal purposes. Cleaners that contain solvents include spot removers, rug cleaners, sanitizers, drain cleaners, and all-purpose cleaners. Some are water-emulsion waxes that will damage wood and cork products. levulinic, acetic, hydroxyacetic (glycolic), Caustic soda/sodium hydroxide (lye), ammonia, Contents of publications may be freely reproduced for educational purposes. Alkaline cleaners are composed of alkali salts, such as sodium bicarbonate (baking soda), sodium carbonate (also known as washing soda or soda ash), sodium metasilicate, and trisodium phosphate (TSP). In the home, borax is used as a natural laundry booster; multipurpose cleaner for woodwork, walls, sinks, and carpets; deodorizer; and disinfectant. Reserve powerful alkalis only when needed. By EPA standards, these products must destroy 99.999% of pathogens within 30 seconds. They can be used on sensitive floor surfaces and will not damage floor finishes. Sarah Jackson has been writing freelance for almost four years, the majority of her work being featured on Adventure Journey, an online travel publication. Heat up 2 cups of water in a regular pan. Mixed with water, this solution is used on areas that require a mild cleaning. Simply put, no. Pacifiers and toys that children may place in their mouths can be sanitized for safety. Pine oil cleaners are all-purpose cleaners made from a natural resin distilled from pine trees.

Work in a well-ventilated area and avoid inhaling products. Be careful to not spray in your face, especially your eyes! Pour all of the water into the spray bottle so that it combines with the rest of your cleaner. Wear protective gloves, eye protection, and long-sleeved shirts and pants while using this product. This is because the dispersing agent within the alkaline cleaner resists any dissolved dirt, preventing any latent attack on the metal. Use caution when handling strong alkaline cleaners as they are caustic and can harm eyes and skin, and should not be inhaled. Diluted solutions are commonly found in household cleaning products. No, vinegar is a mild organic acid with a pH of 23. An edible weed, purslane is packed with vitamins, minerals, antioxidants, and omega-3 fatty acids. They can be very dangerous if swallowed or if the fumes are inhaled. Solvents are cleaning chemicals that dissolve grease, oil, and oily dirt. GB 800 9238 52. Examples of Acids in Household Cleaners. Installing childproof latches on cabinets can prevent children from opening them and possibly ingesting dangerous chemicals. They may be corrosive, meaning they can eat away at metal surfaces or human tissue. These work to inhibit corrosion, and prevent the buildup of calcium and magnesium in water.. Read More Best Stick Blender for Soap Making: 9 Features To Look For. A detergent is a chemical substance used to break up and remove grease and grime. Most people like the way they smell and the fact that they can clean and deodorize at the same time. Products containing flammable liquids should never be used near open flames, including pilot lights on kitchen ranges or gas clothes dryers, furnaces, or lit cigarettes. When using hydrochloric acid, be careful to not let the cleaner come in contact with eyes and skin. Those toxic traces are then breathed in--or even accidentally ingested--by those who live where they've been used. at NMSU and her M.S. Phosphates are a type of builder commonly found in detergent products. These products are non-flammable. Alkaline cleaners include soluble salts ranging from mild (baking soda) to strong (washing soda). Table 3. If no other person is close by and you are hurt or starting to feel sick, then do the following. These types of cleaners are used to unclog sink drains or in the bathroom. Use an alkaline cleaning agent to do the following: And, lets not forget about mild soaps, sometimes referred to as neutral washing agents. Would you like more timeless tips via email? Trisodium phosphate (TSP) is not commonly found in products because most phosphates have been phased out of cleaning products due to environmental concerns. It is best for general household cleaning on surfaces that can tolerate a strong, acidic product. Sonja Koukel is a Professor and Extension Community and Environmental Health Specialist in the Department of Extension Family and Consumer Sciences. The best practice is to put these products in a place that children cannot reach. These include disease-causing strains of salmonella and staph bacteria. Do not leave aerosol (pressurized) containers on a kitchen range, radiator, or furnace; in direct sunlight; or near other heat sources. Takeaway: When cleaning at home, its best to customize the strength of the cleaner to the severity of the mess. Citric acid is a natural substance found in lemons, limes, oranges, and grapefruits. Acids are used to remove mineral deposits, rust stains, and hard water deposits. Box 30001 | Las Cruces, NM 88003-8001, 2022 New Mexico State University - Board of Regents. Alkaline detergents produce a number of chemical and physical actions, including: Compared to organic chlorinated solvents, alkaline cleaners put up an extremely strong case. Consumers must take care when choosing and using acids for cleaning. When the gas is dissolved in water it is called liquid ammonia. Not all floor waxes contain spirit solvents. When mixed in water, pine oil cleaners do not dissolve, but instead make a milky soap. When surfaces are damaged in this way, they soil faster and stain deeper. Yes, with a pH of roughly 13.5 bleach is a strong alkaline disinfectant. This is the worst place to store household cleaners.
 Marketing and Communications | MSC 3K, P.O. In commercial products, phosphoric acid is found in tub, tile, sink, and toilet bowl cleaners. It is environmentally sensitive, does not contain petroleum solvents and is suitable for hydrocarbon-free cleaning. When hes not tinkering in the garden, or fixing something around the house, you can find him working on a vast array of random side projects. Table 2. Rosehips are full of vitamin C and antioxidants and are used for a wide range of medicinal purposes. Cleaners that contain solvents include spot removers, rug cleaners, sanitizers, drain cleaners, and all-purpose cleaners. Some are water-emulsion waxes that will damage wood and cork products. levulinic, acetic, hydroxyacetic (glycolic), Caustic soda/sodium hydroxide (lye), ammonia, Contents of publications may be freely reproduced for educational purposes. Alkaline cleaners are composed of alkali salts, such as sodium bicarbonate (baking soda), sodium carbonate (also known as washing soda or soda ash), sodium metasilicate, and trisodium phosphate (TSP). In the home, borax is used as a natural laundry booster; multipurpose cleaner for woodwork, walls, sinks, and carpets; deodorizer; and disinfectant. Reserve powerful alkalis only when needed. By EPA standards, these products must destroy 99.999% of pathogens within 30 seconds. They can be used on sensitive floor surfaces and will not damage floor finishes. Sarah Jackson has been writing freelance for almost four years, the majority of her work being featured on Adventure Journey, an online travel publication. Heat up 2 cups of water in a regular pan. Mixed with water, this solution is used on areas that require a mild cleaning. Simply put, no. Pacifiers and toys that children may place in their mouths can be sanitized for safety. Pine oil cleaners are all-purpose cleaners made from a natural resin distilled from pine trees.
Marketing and Communications | MSC 3K, P.O. In commercial products, phosphoric acid is found in tub, tile, sink, and toilet bowl cleaners. It is environmentally sensitive, does not contain petroleum solvents and is suitable for hydrocarbon-free cleaning. When hes not tinkering in the garden, or fixing something around the house, you can find him working on a vast array of random side projects. Table 2. Rosehips are full of vitamin C and antioxidants and are used for a wide range of medicinal purposes. Cleaners that contain solvents include spot removers, rug cleaners, sanitizers, drain cleaners, and all-purpose cleaners. Some are water-emulsion waxes that will damage wood and cork products. levulinic, acetic, hydroxyacetic (glycolic), Caustic soda/sodium hydroxide (lye), ammonia, Contents of publications may be freely reproduced for educational purposes. Alkaline cleaners are composed of alkali salts, such as sodium bicarbonate (baking soda), sodium carbonate (also known as washing soda or soda ash), sodium metasilicate, and trisodium phosphate (TSP). In the home, borax is used as a natural laundry booster; multipurpose cleaner for woodwork, walls, sinks, and carpets; deodorizer; and disinfectant. Reserve powerful alkalis only when needed. By EPA standards, these products must destroy 99.999% of pathogens within 30 seconds. They can be used on sensitive floor surfaces and will not damage floor finishes. Sarah Jackson has been writing freelance for almost four years, the majority of her work being featured on Adventure Journey, an online travel publication. Heat up 2 cups of water in a regular pan. Mixed with water, this solution is used on areas that require a mild cleaning. Simply put, no. Pacifiers and toys that children may place in their mouths can be sanitized for safety. Pine oil cleaners are all-purpose cleaners made from a natural resin distilled from pine trees.  Work in a well-ventilated area and avoid inhaling products. Be careful to not spray in your face, especially your eyes! Pour all of the water into the spray bottle so that it combines with the rest of your cleaner. Wear protective gloves, eye protection, and long-sleeved shirts and pants while using this product. This is because the dispersing agent within the alkaline cleaner resists any dissolved dirt, preventing any latent attack on the metal. Use caution when handling strong alkaline cleaners as they are caustic and can harm eyes and skin, and should not be inhaled. Diluted solutions are commonly found in household cleaning products. No, vinegar is a mild organic acid with a pH of 23. An edible weed, purslane is packed with vitamins, minerals, antioxidants, and omega-3 fatty acids. They can be very dangerous if swallowed or if the fumes are inhaled. Solvents are cleaning chemicals that dissolve grease, oil, and oily dirt. GB 800 9238 52. Examples of Acids in Household Cleaners. Installing childproof latches on cabinets can prevent children from opening them and possibly ingesting dangerous chemicals. They may be corrosive, meaning they can eat away at metal surfaces or human tissue. These work to inhibit corrosion, and prevent the buildup of calcium and magnesium in water.. Read More Best Stick Blender for Soap Making: 9 Features To Look For. A detergent is a chemical substance used to break up and remove grease and grime. Most people like the way they smell and the fact that they can clean and deodorize at the same time. Products containing flammable liquids should never be used near open flames, including pilot lights on kitchen ranges or gas clothes dryers, furnaces, or lit cigarettes. When using hydrochloric acid, be careful to not let the cleaner come in contact with eyes and skin. Those toxic traces are then breathed in--or even accidentally ingested--by those who live where they've been used. at NMSU and her M.S. Phosphates are a type of builder commonly found in detergent products. These products are non-flammable. Alkaline cleaners include soluble salts ranging from mild (baking soda) to strong (washing soda). Table 3. If no other person is close by and you are hurt or starting to feel sick, then do the following. These types of cleaners are used to unclog sink drains or in the bathroom. Use an alkaline cleaning agent to do the following: And, lets not forget about mild soaps, sometimes referred to as neutral washing agents. Would you like more timeless tips via email? Trisodium phosphate (TSP) is not commonly found in products because most phosphates have been phased out of cleaning products due to environmental concerns. It is best for general household cleaning on surfaces that can tolerate a strong, acidic product. Sonja Koukel is a Professor and Extension Community and Environmental Health Specialist in the Department of Extension Family and Consumer Sciences. The best practice is to put these products in a place that children cannot reach. These include disease-causing strains of salmonella and staph bacteria. Do not leave aerosol (pressurized) containers on a kitchen range, radiator, or furnace; in direct sunlight; or near other heat sources. Takeaway: When cleaning at home, its best to customize the strength of the cleaner to the severity of the mess. Citric acid is a natural substance found in lemons, limes, oranges, and grapefruits. Acids are used to remove mineral deposits, rust stains, and hard water deposits. Box 30001 | Las Cruces, NM 88003-8001, 2022 New Mexico State University - Board of Regents. Alkaline detergents produce a number of chemical and physical actions, including: Compared to organic chlorinated solvents, alkaline cleaners put up an extremely strong case. Consumers must take care when choosing and using acids for cleaning. When the gas is dissolved in water it is called liquid ammonia. Not all floor waxes contain spirit solvents. When mixed in water, pine oil cleaners do not dissolve, but instead make a milky soap. When surfaces are damaged in this way, they soil faster and stain deeper. Yes, with a pH of roughly 13.5 bleach is a strong alkaline disinfectant. This is the worst place to store household cleaners.
Work in a well-ventilated area and avoid inhaling products. Be careful to not spray in your face, especially your eyes! Pour all of the water into the spray bottle so that it combines with the rest of your cleaner. Wear protective gloves, eye protection, and long-sleeved shirts and pants while using this product. This is because the dispersing agent within the alkaline cleaner resists any dissolved dirt, preventing any latent attack on the metal. Use caution when handling strong alkaline cleaners as they are caustic and can harm eyes and skin, and should not be inhaled. Diluted solutions are commonly found in household cleaning products. No, vinegar is a mild organic acid with a pH of 23. An edible weed, purslane is packed with vitamins, minerals, antioxidants, and omega-3 fatty acids. They can be very dangerous if swallowed or if the fumes are inhaled. Solvents are cleaning chemicals that dissolve grease, oil, and oily dirt. GB 800 9238 52. Examples of Acids in Household Cleaners. Installing childproof latches on cabinets can prevent children from opening them and possibly ingesting dangerous chemicals. They may be corrosive, meaning they can eat away at metal surfaces or human tissue. These work to inhibit corrosion, and prevent the buildup of calcium and magnesium in water.. Read More Best Stick Blender for Soap Making: 9 Features To Look For. A detergent is a chemical substance used to break up and remove grease and grime. Most people like the way they smell and the fact that they can clean and deodorize at the same time. Products containing flammable liquids should never be used near open flames, including pilot lights on kitchen ranges or gas clothes dryers, furnaces, or lit cigarettes. When using hydrochloric acid, be careful to not let the cleaner come in contact with eyes and skin. Those toxic traces are then breathed in--or even accidentally ingested--by those who live where they've been used. at NMSU and her M.S. Phosphates are a type of builder commonly found in detergent products. These products are non-flammable. Alkaline cleaners include soluble salts ranging from mild (baking soda) to strong (washing soda). Table 3. If no other person is close by and you are hurt or starting to feel sick, then do the following. These types of cleaners are used to unclog sink drains or in the bathroom. Use an alkaline cleaning agent to do the following: And, lets not forget about mild soaps, sometimes referred to as neutral washing agents. Would you like more timeless tips via email? Trisodium phosphate (TSP) is not commonly found in products because most phosphates have been phased out of cleaning products due to environmental concerns. It is best for general household cleaning on surfaces that can tolerate a strong, acidic product. Sonja Koukel is a Professor and Extension Community and Environmental Health Specialist in the Department of Extension Family and Consumer Sciences. The best practice is to put these products in a place that children cannot reach. These include disease-causing strains of salmonella and staph bacteria. Do not leave aerosol (pressurized) containers on a kitchen range, radiator, or furnace; in direct sunlight; or near other heat sources. Takeaway: When cleaning at home, its best to customize the strength of the cleaner to the severity of the mess. Citric acid is a natural substance found in lemons, limes, oranges, and grapefruits. Acids are used to remove mineral deposits, rust stains, and hard water deposits. Box 30001 | Las Cruces, NM 88003-8001, 2022 New Mexico State University - Board of Regents. Alkaline detergents produce a number of chemical and physical actions, including: Compared to organic chlorinated solvents, alkaline cleaners put up an extremely strong case. Consumers must take care when choosing and using acids for cleaning. When the gas is dissolved in water it is called liquid ammonia. Not all floor waxes contain spirit solvents. When mixed in water, pine oil cleaners do not dissolve, but instead make a milky soap. When surfaces are damaged in this way, they soil faster and stain deeper. Yes, with a pH of roughly 13.5 bleach is a strong alkaline disinfectant. This is the worst place to store household cleaners.